Home > About Us
About Us
TVAX Biomedical is a clinical stage development company advancing its novel targeted cell-based immunotherapy for the treatment of cancer.
Current treatment options for cancer, which include surgery, radiation and/or chemotherapy, are not adequately meeting patient treatment needs. Radiation and chemotherapy in particular are highly toxic and rarely “cure” the patient by permanently eliminating the cancer. With more than 1.5 million new cases of cancer diagnosed and more than 500,000 cancer deaths in the U.S. each year, more effective, less toxic treatment options are desperately needed.
TVAX Immunotherapy Platform
TVAX’s immunotherapeutic cancer treatment platform is built upon more than 50 years of intensive scientific and medical research into the interactions between cancer and the immune system. This novel, proprietary immunotherapy platform has demonstrated the potential to effectively treat numerous cancers with very low toxicity, potentially offering a paradigm shift in the systemic treatment of cancer.
Lead Clinical Candidates
The company’s lead candidates, which are focused on treating brain and kidney cancer, are supported by positive Phase 2 clinical data, as well as extensive preclinical and Phase 1 safety studies. Based on these supportive data, the US Food and Drug Administration (FDA) has authorized Fast Track Designation for TVAX Biomedical to perform a Phase 2b clinical trial to test TVI-Brain-1 as a treatment for glioblastoma. In addition to these lead programs, extensive proof-of-concept and safety data have been generated to support the use of TVAX Immunotherapy in several other cancer treatment indications.
The Advantage of TVAX Immunotherapy
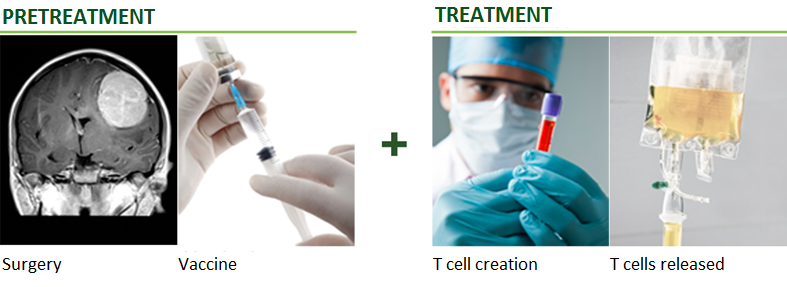
The key distinction between TVAX and other cancer immunotherapy companies is that TVAX Immunotherapy uses BOTH cancer vaccine pretreatment to generate cancer-specific T cells AND activated “killer” T cell treatment – this proprietary combination has demonstrated significant efficacy.
TVAX’s proprietary treatment has received orphan product designation from FDA for the treatment of any primary central nervous system malignancy, providing the company with seven years of marketing exclusivity following FDA marketing approval.